The antenna complexes in the outer membranes of photosynthetic bacteria are efficient systems designed to absorb (“harvest”) light energy and funnel it towards a reaction center where it is used to effect a charge separation and generate a proton gradient across the membrane. This proton gradient is then used to drive the synthesis of ATP to complete the photosynthetic conversion of radiation into chemically stored energy. Single-molecule studies of the light-harvesting 2 (LH2) complex of the photosynthetic purple bacterium Rhodopseudomonas acidophila have helped to improve our understanding of the photophysics which are involved this important biological process.
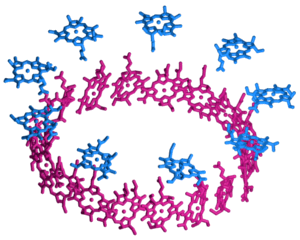
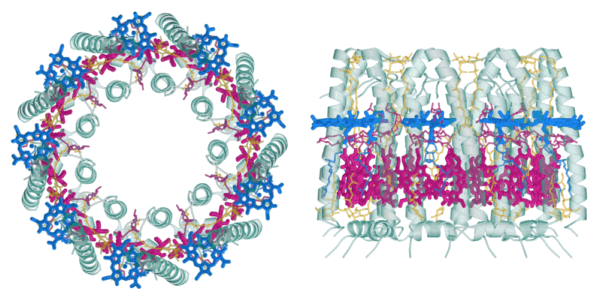
The studied LH2 complex consists of 27 bacteriochlorophyll a (BChl a) cofactors which are organized in two substructures, the B800 and the B850 ring. This is illustrated in the figure to the right which gives a schematic representation of their arrangement, as determined from the X-ray structure [1]. The B800 ring (blue) consists of 9 BChl a molecules in a geometry with a 9-fold symmetry axis which coincides with the overall axis of the cylindrical complex; the individual pigments are well separated with their molecular planes perpendicular to this C9 axis. The 18 chromophores of the B850 ring (red), on the other hand, are spaced more closely with their planes roughly parallel to the common C9 axis, somewhat similar to the arrangement of the blades around the axle of a turbine. The image below shows two views of the complete complex to illustrate how the BChl a cofactors are embedded in the barrel-shaped protein scaffold (green) which also contains the auxiliary carotenoid chromophores (yellow).
The difference in dipole-dipole coupling between the B800 and the B500 cofactors and its effects on energy transfer are nicely illustrated by low-temperature single-complex spectroscopy: The B800 pigments are weakly coupled, therefore it is possible to observe well-separated fluorescence excitation lines in the B800 band of individual LH2 complexes at 1.2 K. A rotation of the excitation polarization furthermore shows that the lines correspond to largely localized excitations with their polarization dependent intensity reflecting the different orientations of the individual pigment transition dipoles [2,3]. The B850 band, on the other hand, has to be described in terms of the exciton model: Here the coupling between the BChl a molecules is so strong that it gives rise to excitonic states coherent over the whole ring. These states are sensitive to variations in the transition energies of the B850 chromophores (diagonal disorder) and to variations in the coupling strengths for different pairs of chromophores (off-diagonal disorder). Low-temperature fluorescence excitation spectra provide direct access to the energy splitting, polarization dependence and relative oscillator strengths of these excitonic states [4]. The distributions of these parameters reflect the disorder in the complexes and it could be shown that the results are best explained by assuming an elliptical distortion of the complexes [4-7].
In addition to their important biological functions, antenna complexes are the first example of a strongly coupled system of a few chromophores that can be studied individually. Their new photophysical properties (as compared to the monomers) illustrate the wealth of novel effects that can be expected or designed in molecular aggregates and assemblies.
References
- G. McDermott, S.M. Prince, A.A. Freer, A.M. Hawthornthwaite-Lawless, M.Z. Papiz,
R.J. Cogdell, N.W. Isaacs
“Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria”
Nature 374 (1995) 517 – 521 - A.M. van Oijen, M. Ketelaars, J. Köhler, T.J. Aartsma, J. Schmidt
“Spectroscopy of single light-harvesting complexes from purple photosynthetic bacteria at 1.2 K”
J. Phys. Chem. B 102 (1998) 9363-9366 - A.M. van Oijen, M. Ketelaars, J. Köhler, T.J. Aartsma, J. Schmidt
“Spectroscopy of individual LH2 complexes of Rhodopseudomonas acidophila: localized excitations in the B800 band”
Chem. Phys. 247 (1999) 53-60 - A.M. van Oijen, M. Ketelaars, J. Köhler, T.J. Aartsma, J. Schmidt
“Unraveling the electronic structure of individual photosynthetic pigment-protein complexes”
Science 285 (1999) 400-402 - A.M. van Oijen, M. Ketelaars, J. Köhler, T.J. Aartsma, J. Schmidt
“Spectroscopy of individual LH2 complexes of Rhodopseudomonas acidophila: Diagonal disorder, intercomplex heterogeneity, spectral diffusion and energy transfer in the B800 band”
Biophys. J. 78 (2000) 1570-1577 - M. Ketelaars, A.M. van Oijen, M. Matsushita, J. Köhler, J. Schmidt, T.J. Aartsma
“Spectroscopy on the B850 band of individual light-harvesting 2 complexes of Rhodopseudomonas acidophila: I. Experiments and Monte-Carlo simulations”
Biophys. J. 80 (2001) 1591-1603 - M. Matsushita, M. Ketelaars, A.M. van Oijen, J. Köhler, T.J. Aartsma, J. Schmidt
“Spectroscopy on the B850 band of individual light-harvesting 2 complexes of Rhodopseudomonas acidophila: II. Exciton states of an elliptically deformed ring aggregate”
Biophys. J. 80 (2001) 1604-1614 - The images of the light harvesting 2 complex of Rhodopseudomonas acidophila were generated from the Protein Data Bank record 1KZU using MolScript and Raster3D.